You can Download Chapter 15 Polymers Questions and Answers, Notes, 2nd PUC Chemistry Question Bank with Answers Karnataka State Board Solutions help you to revise complete Syllabus and score more marks in your examinations.
Karnataka 2nd PUC Chemistry Question Bank Chapter 15 Polymers
2nd PUC Chemistry Polymers NCERT Textbook Questions
Question 1.
Explain the terms polymer and monomer.
Answer:
Polymers are high molecular mass of macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass (103-107u). In a polymer, various monomer units are joined by strong covalent bonds. Polymers can be natural as well as synthetic. Polythene, rubber and nylon 6,6 are examples of polymers.
Monomers are simple, reactive molecules that combine with each other in large numbers through covalent bonds to give rise to polymers. For example ethene, propene, styrene, vinyl chloride.
Question 2.
What are natural and synthetic polymers? Give two examples of each type.
Answer:
Natural polymers are polymers that are found in nature. They are formed by plants and animals. Examples include protein, cellulose, starch etc.
Synthetic polymers are polymers made by human beings Examples include plastic (polythene), synthetic fibers (nylon 6, 6), synthetic rubbers (Buna-S).
![]()
Question 3.
Distinguish between the terms homopolymer and copolymer and give an example of each.
Answer:
| Homopolymer | Copolymer |
| The polymers that are formed by the polymerization of a single monomer are known as homopolymer. In other words, the repeating units of homopolymers are derived only from one monomer. For example, polythene is a homopolymer of ethane. | The polymers whose repeating units are derived from two types of monomers are known as copolymers. For example, Buna-S is a copolymer of 1, 3 – butadiene and stryrene. |
Question 4.
How do you explain the functionality of a monomer?
Answer:
The functionality of a monomer is the number of binding sites that is/are present in that monomer.
For example, the functionality of monomers such as ethene and propene is one and that of 1, 3-butadiene and adipic acid is two.
Question 5.
Define the term polymerisation.
Answer:
Polymerisation is the process of forming high molecular mass (103-107u) macromolecules, which consist of repeating structural units derived from monomers. In a polymer, various monomer units are joined by strong covalent bonds.
Question 6.
Is ( NH-CHR-CO )8, a homopolymer or copolymer?
Answer:
(NH CHR-CO)8 is a homopolymer because it is obtained from a single monomer unit, NH2-CHR-COOH.
Question 7.
In which classes, the polymers are classified on the basis of molecular forces?
Answer:
On the basis of magnitude of intermolecular forces present in polymers, they are classified into the following groups:
- Elastomers
- Fibers
- Thermoplastic polymers
- Thermosetting polymers
Question 8.
How can you differentiate between addition and condensation polymerisation?
Answer:
Addition polymerisation is the process of repeated addition of monomers, possessing double or triple bonds to form polymers. For example, polythene is formed by addition polymerization of ethene
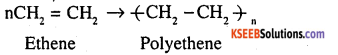
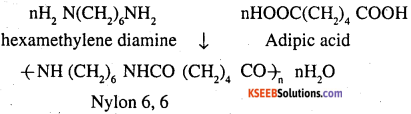
Condensation polymerization is the process Of formation of polymers by repeated condensation reactions between two different bi functional or tri functional monomers. A small molecule such as water or hydrochloric acid is eliminated in each condensation. For example nylon 6, 6 is formed by condensation polymerisation of hexamethylene diamine and adipic acid.
![]()
Question 9.
Explain the term copolymerisation and give two examples.
Answer:
Formation of polymers from two or more different monomeric units is called copolymerization. Multiple units of each monomer are present in a copolymer. The process of forming polymer BunaS from 1, 3-butadiene and styrene is an example of copolymerization.
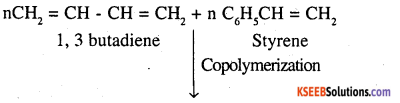
Nylon 6,6 is also a copolymer formed by hexamethylene diamine and adipic acid.

Question 10.
Write the free radical mechanism for the polymerisation of ethene.
Answer:
Polymerization of ethene to polythene consists of heating or exposing to a light mixture of ethene with a small amount of benzoyl peroxide as the initiator. The reaction involved in this process is given below:
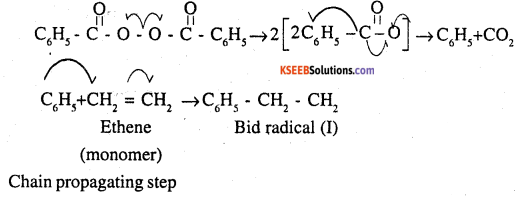
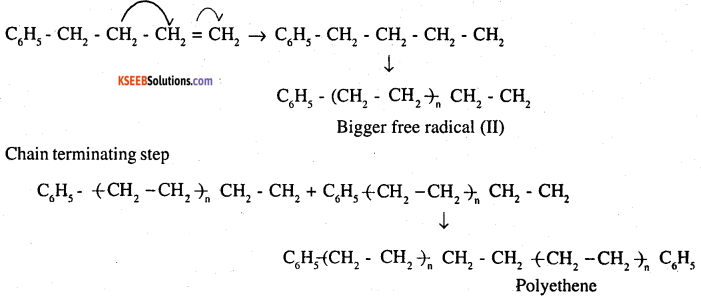
Question 11.
Define thermoplastics and thermosetting polymers with two examples of each.
Answer:
Thermoplastics polymers are linear (slightly branched) long chain polymers, which can be repeatedly softened and hardened on heating. Hence, they can be modified again and again. Examples include polyethene, polystyrene.
Thermosetting polymers are cross-linked or heavily branded polymers which get hardened during moulding process. These plastics cannot be softened again on heating. Examples of thermosetting plastics include bakelite, urea-formaldelyde resins.
Question 12.
Write the monomers used for getting the following polymers.
1. Polyvinyl chloride
2. Teflon
3. Bakelite
Answer:
1. Vinyl chloride (CH2 = CHCI)
2. Tetrafluro ethylene (CF2 = CF2)
3. Formaldehyde (HCHO) and phenol (C6H5OH)
![]()
Question 13.
Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Answer:
One common initiator used in tree radical addition polymerization is benzoyl peroxide. Its structure is given below.
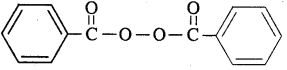
Question 14.
How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Answer:
Natural rubber is a linear cis-polyisoprene in which the double bonds are present C2 and C3 of the isoprene units.
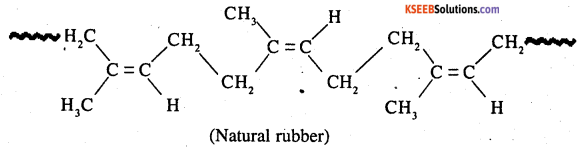
Because of this cis — configuration, inter molecular interactions between the various standard of isoprene are quite weak. As a result, various strands in natural rubber are arranged randomly. Hence it shows elasticity.
Question 15.
Discuss the main purpose of vulcanisation of rubber.
Answer:
Natural rubber though useful has some problems associated with its use. These limitations are discussed below;
- Natural rubber is quite soft and sticky at room temperature. At elevated temperatures (>335K), if becomes even softer. At low temperature (<283K) it becomes brittle. Thus to maintain its elasticity, natural rubber is generally used in the temperature range of 283K-335K.
- It has the capacity to absorb large amounts of water.
- It has low tensile strength and low resistance to abrasion.
- It is soluble in non polar solvents
- It is easily attacked by oxidizing agents
Vulcanisation of natural rubber is done to improve all these properties. In this process, a mixture of raw rubber with sulphur and appropriate additive is heated at a temperature range between 373K and 415K.
Question 16.
What are the monomeric repeating units of Nylon-6 and Nylon-6,6?
Answer:
The monomeric repeating unit of nylon 6 is [NH – (CH2)5 – CO], which is derived form caprolactum.
The monomeric repeating unit of nylon 6, 6 is [NH – (CH2)6 – NH – CO – (CH2)4 – CO], which is derived from hexamethylene diamine and adipic acid.
Question 17.
Write the names and structures of the monomers of the following polymers:
(i) Buna-S
(ii) Buna-N
(iii) Dacron
(iv) Neoprene
Answer:
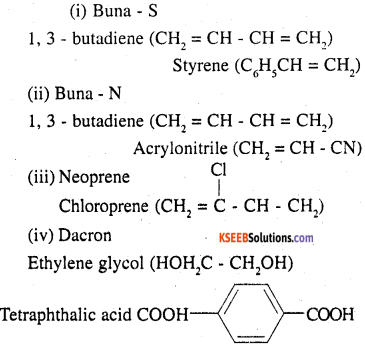
![]()
Question 18.
Identify the monomer in the following polymeric structures.
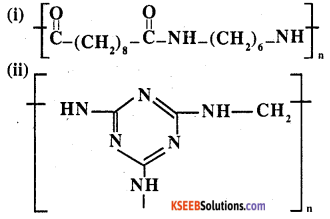
Answer:
Monomers are:
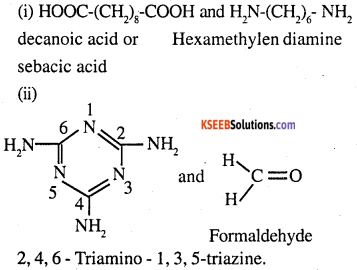
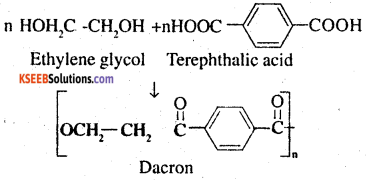
Question 19.
How is dacron obtained from ethylene glycol and terephthalic acid ?
Answer:
The condensation polymerisation of ethylene glycol and terephthalic acid leads to the formation dacron.
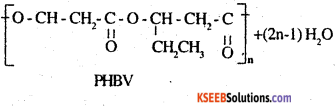
Question 20.
What is a biodegradable polymer ? Give an example of a biodegradable aliphatic polyester.
Answer:
A polymer that can be decomposed by bacteria is called a biodegradable polymer. Poly – β- hydroxybutyrate – Co -β – hydroxyvalerate (PHBV) is a biodegradable aliphatic polyester.
2nd PUC Polymers Additional Questions
Question 1.
How are polymers classified on the basis of solution?
Answer:
- Linear polymers such as high density polythene (HDP), Polyvinyl chloride, nylons, polyester etc.
- Branched polymers such as low density polythene (LDP), amylopectin, glycogen etc.
Question 2.
What is the difference between Buna-S and Buna-N ?
Answer:
Both are copolymers basically Buna-N is a copolymer of
1,3- butadiene and acrylonitrile. Buna-S is a copolymer of
1,3- butadiene and styrene.
Question 3.
What are the monomeric units of Nylon 6 and Nylon 6, 6?
Answer:
Monomeric repeating unit of Nylon 6 :
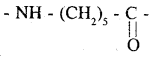
which is derived from caprolactam monomeric repeating unit of Nylon 6,6 :
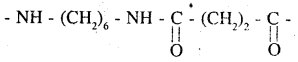
which is derived from 2 monomers, hexamethylenediamine and adipic acid and has the following structure.
Question 4.
What are the disadvantages of natural rubber which are compromised by vulcanisation ?
Answer:
- It becomes more soft and sticky at high temperatures and brittle at low temperatures.
- It. has large water absorption capacity and low tensile strength and low resistance to abrasion.
- Not resistant to action of organic solvents.
- Easily attacked by oxygen and other oxidising agents.
![]()
Question 5.
What does PMMA stand for?
Answer:
Poly(methyl methacrylate)
Question 6.
How are polymers classified on the basis of molecular forces?
Answer:
- Elastomers
- Fibres
- Thermoplastic
- Thermosetting.
Question 7.
What is copolymerisation (and give an example)?
Answer:
When 2 or more different monomers are allowed to polymerise together, product is called copolymer and process is called copolymerisation.
Ex: Buna-S: 1, 3 – butadiene and styrene.
Question 8.
What thermoplastic and thermosetting polymers ?
Answer:
Thermoplastics are linear polymers which can be repeatedly softened on heating and hardened on cooling and hence can.be repeatedly used.
ex: polythene, polypropene etc.
Thermosetting polymers are on the other hand permanently setting polymers. On heating, they harden and cant be softened again.
ex: bakelite, urea-formaldehyde resin.
Question 9.
What is functionality of a polymer?
Answer:
It is the number of bonding sites in a molecule.
Functionality of styrene is one, whereas it is for adipic acid.
Question 10.
Write free radical mechanism for polymerisation of ethene.
Answer:
Initiation:
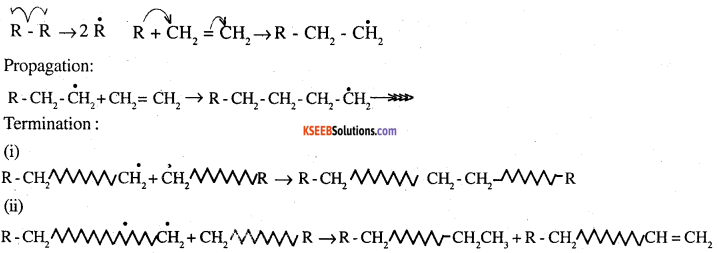
![]()