You can Download KSEEB Solutions for Class 8 Science Chapter 4 Materials: Metals and Non-Metals Questions and Answers Pdf, Notes help you to revise the complete syllabus.
KSEEB Solutions for Class 8 Science Chapter 4 Materials: Metals and Non-Metals
Materials: Metals and Non-Metals Textbook Questions and Answers
Question 1.
Which of the following can be beaten into thin sheets:
(a) Zinc
(b) Phosphorus
(c) Sulphur
(d) Oxygen
Answer:
(a) Zinc
Question 2.
Which of the following statement is correct?
(a) All metals are ductile.
(b) All non-metals are ductile.
(c) Generally, metals are ductile.
(d) Some non-metals are ductile.
Answer:
(d) Generally, metals are ductile.
![]()
Question 3.
Fill in the blanks:
- Phosphorus is a very _______ non-metal.
- Metals are _______ conductors of heat and __________
- Iron is _______ reactive than copper.
- Metals react with acid to produce __________ gas.
Answer:
- reactive
- good, electricity
- more
- hydrogen
Question 4.
Mark ‘T’ if the statement is true and ‘F’ if it is false.
- Generally, non-metals react with acids.
- Sodium is a very reactive metal.
- Copper displaces zinc from zinc sulphate solution.
- Carbon can be drawn into wires.
Answer:
- False
- True
- False
- False
Question 5.
Some properties are listed in the following table. Distinguish between metals and non-metals on the basis of these properties:
| Properties | Metals | Non-metals |
| 1. Appearance | ||
| 2. Hardness | ||
| 3. Malleability | ||
| 4. Ductility | ||
| 5. Heat conduction | ||
| 6. Conduction of electricity |
Answer:
| Properties | Metals | Non-metals |
| 1. Appearance | Lustrous (shiny) | Non-lustrous (dull) |
| 2. Hardness | Very hard | Soft |
| 3. Malleability | Malleable | Not malleable |
| 4. Ductility | Ductile | Not ductile |
| 5. Heat conduction | Good conductors | Bad conductors of heat |
| 6. Conduction of electricity | Good conductor of electricity | Bad conductors of electricity |
Question 6.
Give reasons for the following:
- Aluminium foils are used to wrap food items.
- Immersion rods for heating liquids are made up of metallic substances.
- Copper cannot displace zinc from its salt solution.
- Sodium and potassium are stored in kerosene.
Answer:
- Aluminium is non-toxic and malleable and hence thin sheets of aluminium can be formed.
- Metallic substances are good conductors of electricity therefore immersion rods are made up of metallic substances.
- Copper is less reactive than zinc and hence it cannot displace zinc from its salt solution.
- Sodium and potassium are stored in kerosene because it vigorously reacts with oxygen and water.
![]()
Question 7.
Can you store the lemon pickle in an aluminium utensil? Explain.
Answer:
No, we can not store the lemon pickle in an aluminium utensil because aluminium is metal and thus will react with lemon and produce toxic substances.
Question 8.
Match the substance given in Column A with uses given in Column B.
| A | B |
| (i) Gold | (a) Thermometers |
| (ii) Iron | (b) Electric wire |
| (iii) Aluminium | (c) Wrapping food |
| (iv) Carbon | (d) Jewellery |
| (v) Copper | (e) Machinery |
| (vi) Mercury | (f) Fuel |
Answer:
| A | B |
| (i) Gold | (d) Jewellery |
| (ii) Iron | (e) Machinery |
| (iii) Aluminium | (c) Wrapping food |
| (iv) Carbon | (f) Fuel |
| (v) Copper | (b) Electric wire |
| (vi) Mercury | (a) Thermometers |
Question 9.
What happens when:
(i) Dilute sulphuric acid is poured on a copper plate.
(ii) Iron nails are placed in a copper sulphate solution. Write word equations of the reactions involved.
Answer:
(i) Copper reacts with sulphuric acid and forms copper sulphate and hydrogen (gas).
Copper + Sulphuric acid → Copper sulphate + Hydrogen gas.
Cu + H2SO4 → CuSO4 + H2 ↑
(ii) Iron displaces copper from copper sulphate solution hence a reddish-brown copper is formed on the iron nails and the blue colour of copper sulphate solution slowly becomes light green.
Iron + Copper sulphate → Copper + Iron sulphate.
Fe + CuSO4 → Cu + FeSO4
Question 10.
Saloni took a piece of burning charcoal and collected the gas evolved in a test tube:
(i) How will she find the nature of the gas?
(ii) Write down word equations of all the reactions taking place in this process.
Answer:
(i) To find the nature of the gas, she will dissolve it in the water and test it with litmus paper.
(ii) (a) Charcoal (c) + Oxygen (O2) → Carbon dioxide (CO2)
(b) Carbon dioxide (CO2) + Water (H2O) → Carbonic acid (H2CO3)
The nature of this gas is acidic because it turns red litmus blue.
![]()
Question 11.
One day Reeta went to a jeweler’s shop with her mother. Her mother gave old gold jewellery to the goldsmith to polish. The next day when they brought the jewellery back they found that there was a loss in its weight. Can you suggest a reason for the loss of weight?
Answer:
The jeweller must have cleared the jewellery in aqua regia solution. This solution is a mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3 : 1. This acid solution dissolves some gold in it, due to which there is a loss in the weight of jewellery.
Materials: Metals and Non-Metals Additional Questions and Answers
Question 1.
Give examples of metals.
Answer:
Examples of metals are gold, silver, sodium tungsten, cadmium, nickel, uranium, and mercury.
Question 2.
Give examples of non-metals?
Answer:
Examples of non-metals are chlorine, helium, oxygen, carbon, fluorine, sulphur, iodine, and bromine.
Question 3.
Name the metal which is liquid at room temperature.
Answer:
Mercury
![]()
Question 4.
Write the physical properties of metals.
Answer:
The physical properties of metals are:
- Metals are solid at room temperature except for mercury.
- Metals generally have high melting points.
- The surface of most metals has a shiny appearance.
- Metals are malleable, i.e., they can be beaten into sheets.
- Metals are ductile, i.e., they can be stretched into wires.
- Metals are good conductors of electricity.
- Metals are good conductors of heat.
- Metals are sonorous, i.e., they produce sound when they are struck by themselves or by any other object.
Question 5.
(i) What do you observe when magnesium ribbon is heated over a flame?
(ii) What change do you observe in the colour of red and blue litmus papers when they are dipped into solution obtained by mixing solid residue from (i) in water? What do you conclude?
(iii) Write chemical equations for the above two reactions.
Answer:
(i) When magnesium ribbon is heated over the flame then it burns with brilliant light and gets converted into a white solid residue.
(ii) The solution obtained by mixing white solid residue produced above with water turns red litmus into blue colour and blue litmus remains unaffected. This shows that the solution is alkaline hence oxide formed is basic in nature.
(iii) 2Mg + O2 → 2MgO
MgO + H2O → Mg(OH)2
Question 6.
What happens when a small piece of sodium metal is dried and kept in water?
Answer:
When a small piece of sodium metal is dried and kept in the water we observe that the sodium piece starts moving in water with a hissing sound and sodium catches fire.
2Na + 2H2O → 2NaOH + H2 ↑
Question 7.
Does magnesium react with cold water? What happens when the water is heated?
Answer:
Magnesium does not react or mildly react with cold water. When water is heated then magnesium reacts with hot water or steam vigorously and produces magnesium hydroxide and hydrogen.
Mg + 2H2O → Mg(OH)2 + H2
(Magnesium) + Water → Magnesium Hydroxide + Hydrogen
![]()
Question 8.
How do metals react with dilute acids?
Answer:
Metals usually displace hydrogen from dilute acids and salt is formed. Only the less reactive metals like copper, silver, and gold do not displace hydrogen from dilute acids.
Metal + Hydrochloric acid → Metal chloride + Hydrogen (gas)
Metal + Sulphuric acid → Metal sulphate + Hydrogen (gas)
Question 9.
Write the word equation for the reaction of magnesium with dil. hydrochloric acid.
Answer:
Magnesium reacts with dil. hydrochloric acid to form magnesium chloride and hydrogen gas is evolved.
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Question 10.
What happens when iron nails are added to copper sulphate solution?
Answer:
When iron nails are added to copper sulphate solution the blue colour of copper sulphate starts fading and iron sulphate is formed. Iron displaces copper from copper sulphate and copper gets deposited on the iron nails.
Fe + CuSO4 → FeSO4 + Cu
Question 11.
What happens when strips of magnesium are added to iron sulphate solution?
Answer:
When strips of magnesium are added to iron sulphate solution then magnesium displaces iron from iron sulphate solution and forms magnesium sulphate. Iron gets deposited on the magnesium strips.
Mg + FeSO4 → MgSO4 + Fe
Question 12.
What would you observe when a strip of zinc is dipped in the solution of copper sulphate?
Answer:
When a strip of zinc is dipped in the solution of copper sulphate solution then zinc displaces copper from copper sulphate solution. A red-brown coating of copper forms on the strips of zinc.
![]()
Question 13.
Write the uses of metals.
Answer:
The metals are used for:
- making machines
- making buildings
- making automobiles, aeroplanes, trains, satellites, industrial gadgets
- making cooking utensils and water boilers
- making electric gadgets
- making electric cables
- fine electric contacts in computers and solar cells
- for making jewellery, mirrors
- making foils for wrapping of food items, medicines, chocolates, cigarettes, etc.
Question 14.
Why is iron used for making machines, automobiles, and buildings?
Answer:
Iron is very strong, hard, and rigid therefore, it is used for making machines, automobiles, and buildings.
Activities
Activity 1
Take a small iron nail, a coal piece, a piece of thick aluminium wire, and a pencil lead. Beat the iron nail with a hammer (Fig.). Try to hit hard. Hit hard the aluminium wire also. Then repeat the same kind of treatment on the coal piece and pencil lead. Record your observations in the table.

Table: Malleability of Materials
| Object/Material | Change in shape (Flattens/Breaks into pieces) |
| Iron nail | Flattens |
| Coal piece | Breaks into pieces |
| Aluminium | Wire Flattens |
| Pencil lead | Breaks into pieces |
Activity 2
Recall how to make an electric circuit to test whether electricity can pass through an object or not (Fig.).
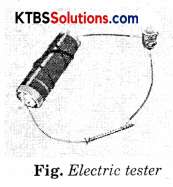
Now, repeat the activity with the materials mentioned in the table. Observe and group these materials into good conductors and poor conductors.
Table: Electrical Conductivity of Materials
| Materials | Good Conductor/Poor Conductor |
| 1. Iron rod/nail | Good Conductor |
| 2. Sulphur | Poor Conductor |
| 3. Coal piece | Poor Conductor |
| 4. Copper wire | Good Conductor |
Activity 3
Let us check the nature of rust formed as a result of the reaction between iron, oxygen, and water. Collect a spoonful of rust and dissolve it in a very little amount of water. You will find that the rust remains suspended in water. Shake the suspension well. Test the solution with red and blue litmus papers (Fig.).
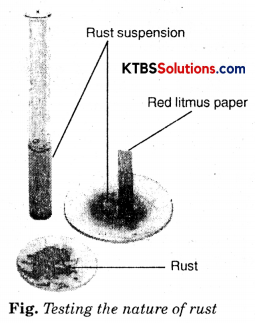
What do you observe? Is the solution acidic or basic?
Answer:
We observe that red litmus turns blue. The solution is basic.
![]()
Activity 4
Take a small amount of powdered sulphur in a deflagrating spoon and heat it. If the deflagrating spoon is not available, you may take a metallic cap of any bottle and wrap a metallic wire around it and give it the shape shown in Fig. (a).
As soon as sulphur starts burning, introduce the spoon into a gas jar/glass tumbler [Fig.(a)]. Cover the tumbler with a lid to ensure that the gas produced does not escape. Remove the spoon after some time. Add a small quantity of water into the tumbler and quickly replace the lid. Shake the tumbler well. Check the solution with red and blue litmus papers [Fig. (b)].
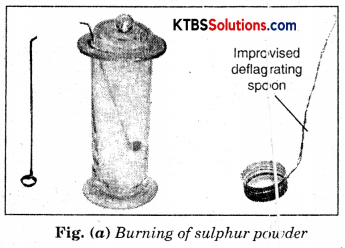
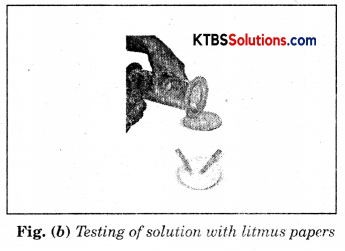
Question (i) What do you observe?
Answer:
We observe that blue litmus turns red. Therefore, this solution is acidic.
Activity 5
Take a 250 ml beaker/glass tumbler. Fill half of it with water. Now carefully cut a small piece of sodium metal. Dry it using filter paper and wrap it in a small piece of cotton. Put the sodium piece wrapped in cotton into the beaker. Observe carefully. When the reaction stops touch the beaker. What do you feel? Has the beaker become hot? Test the solution with red and blue litmus papers. Is the solution acidic or basic?

Question (i).
What do you feel? Has the beaker become hot?
Test the solution with red and blue litmus papers. Is the solution acidic or basic?
Answer:
Yes. The beaker becomes very hot. The solution is basic because it turns red litmus blue.
![]()
Activity 6
Take samples of metals and non-metals listed in the table in separate test tubes and label them as A, B, C, D, E, and F. With the help of a dropper add 5 ml of dilute hydrochloric acid to each test tube one by one. Observe the reactions carefully. If no reaction occurs in the cold solution, warm the test tube gently. Bring a burning matchstick near the mouth of each test tube. Repeat the same activity using dilute sulphuric acid instead of dilute hydrochloric acid. Record your observation in the table.
Table 5: Reaction of Metals and Non-metals with Acids
| Test tube Label | Metal/Non-metal | Reaction with Dilute Hydrochloric Acid Room Warm Temperature | Reaction with Dilute sulphuric Acid Room Warm Temperature |
| A | Magnesium (ribbon) | Hydrogen gas is liberated | |
| B | Aluminium (foil) | Hydrogen gas is liberated | |
| C | Iron (filings) | Hydrogen gas is liberated | |
| D | Copper (peeled flexible wire) | No reaction takes place | Hydrogen gas is liberated |
| E | Charcoal (powder) | No reaction takes place | No reaction takes place |
| F | Sulphur (powder) | No reaction takes place | No reaction takes place |
Activity 7
Prepare a fresh solution of sodium hydroxide in a test tube by dissolving 3-4 pellets of it in 5 ml of water. Drop a piece of aluminium foil into it. Bring a burning matchstick near the mouth of the test tube. Observe carefully.
Answer:
We observe that a gas is produced which burns with a pop sound. This gas is hydrogen gas.
Activity 8
Take five 100 ml beakers and label them A, B, C, D, and E. Take about 50 ml of water in each beaker. Dissolve in each beaker a teaspoonful of each substance as indicated in Fig. (a).
Keep the beakers undisturbed for some time. Record your observations in your notebook.

Beaker A: Copper sulphate (CuSO4) + Zinc granule (Zn)
Beaker B: Copper sulphate (CuSO4) + Iron nail (Fe)
Beaker C: Zinc sulphate (ZnSO4) + Copper turnings (Cu)
Beaker D: Iron sulphate (FeSO4) + Copper turnings (Cu)
Beaker E: Zinc sulphate (ZnSO4) + Iron nail (Fe)
Fig. (a) and (b) Displacement reactions.
![]()
Question (i).
What changes do you observe in the various beakers?
Answer:
In beaker A, zinc displaces copper from copper sulphate (CuSO4). The blue colour of copper sulphate disappears and a powdery red mass of copper is deposited at the bottom of the beaker.
Copper sulphate + Zinc → Zinc sulphate + Copper
In beaker B, iron displaces copper from copper sulphate solution. The blue colour of copper sulphate disappears and it becomes light green. A red coloured powder of copper is deposited on the copper.
In beaker C, D, E, there is no change.