You can Download KSEEB Solutions for Class 8 Science Chapter 10 Combustion and Flame Questions and Answers Pdf, Notes help you to revise the complete syllabus.
KSEEB Solutions for Class 8 Science Chapter 10 Combustion and Flame
Combustion and Flame Textbook Questions and Answers
Question 1.
List conditions under which combustion can take place.
Answer:
The conditions for combustion are:
- The presence of a combustible substance.
- The presence of air with a sufficient amount of oxygen.
- The temperature of combustible substances should be more than its ignition temperature.
![]()
Question 2.
Fill in the blanks:
- Burning of wood and coal causes _________ of air.
- A liquid fuel, used in homes is _________
- Fuel must be heated to its _________ before it starts burning.
- Fire produced by oil cannot be controlled by _________
Answer:
- Pollution
- kerosene
- ignition temperature
- water
Question 3.
Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer:
The use of CNG in automobiles has reduced pollution in our cities because CNG burns with a blue smokeless flame and does not leave any ash after burning.
![]()
Question 4.
Compare LPG and wood as fuels.
Answer:
| LPG | Wood |
| 1. It has a more calorific value. | 1. It has less calorific value. |
| 2. It is smoke-free fuel. | 2. It gives out a lot of smoke which is quite dangerous. |
| 3. It is easy to transport. | 3. It is difficult to transport wood. |
| 4. It is easily stored in cylinders. | 4. It is difficult to store as it needs space to store. |
| 5. It leaves no residue. | 5. Ash is produced. |
Question 5.
Give reasons:
(a) Water is not used to control the fire involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Answer:
(a) Water is not used to control the fire involving electrical equipment because water conducts electricity and thus, there is the possibility of the electric current electrocuting the person trying to extinguish the fire with water.
(b) LPG is a better fuel than wood because it is smokeless, does not leave any residue, has a high calorific value, and low ignition temperature.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not because the heat supplied to the paper is transferred to the aluminium pipe so the aluminium lowers the temperature of the paper. In the presence of aluminium, the ignition temperature of paper is not reached. Hence, it does not burn.
![]()
Question 6.
Make a labelled diagram of a candle flame.
Answer:
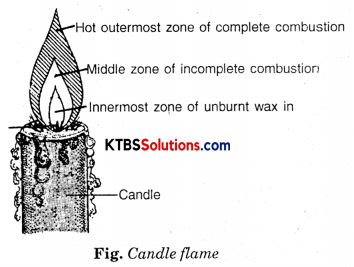
Question 7.
Name the unit in which the calorific value of a fuel is expressed.
Answer:
Kilojoules/kilogram (KJ/Kg).
Question 8.
Explain how CO2 is able to control fires.
Answer:
CO2 forms a large around the burning substance and cuts off the supply of air (oxygen) to it and hence extinguishes fire.
Question 9.
It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Answer:
The ignition temperature of dry leaves is much less than that of green leaves (due to the presence of water). Hence, it is difficult to burn a heap of green leaves but dry leaves catch fire easily.
![]()
Question 10.
Which zone of a flame does a goldsmith use for melting gold and silver and why?
Answer:
A goldsmith uses the outermost zone of a flame for melting gold, and silver because it the hottest zone of the flame.
Question 11.
In an experiment, 4.5 kg of fuel was completely burnt. The heat produced was measured to be 180,000 KJ. Calculate the calorific value of the fuel.
Answer:
The heat produced by burning 4.5kg of a fuel = 1,80,000 KJ
The heat produced by burning 1 kg of a fuel = \(\frac{1,80,000}{4.5}\) = 40,000 KJ/kg.
∴ The caloric value of the fuel is 40,000 KJ/kg.
Question 12.
Can the process of rusting be called combustion? Discuss.
Answer:
The process of rusting can be called combustion because it produces heat. This combustion takes place at a slow rate.
![]()
Question 13.
Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Answer:
The water of Ramesh’s beaker will get heated in a shorter time because the outermost part of the flame is the hottest.
Combustion and Flame Additional Questions and Answers
Question 1.
What are fuels?
Answer:
Fuels are stores of energy which on burning give heat and light.
Question 2.
Name a few fuels used in our homes.
Answer:
Cowdung, wood, coal, LPG, kerosene.
Question 3.
What is combustion?
Answer:
The process of production of heat and light by the burning of a combustible substance in the presence of air is called combustion.
![]()
Question 4.
Write an example to show the process of combustion.
Answer:
Magnesium burns to form magnesium oxide and produces heat and light.
2Mg + O2 → 2MgO + Heat + Light
Question 5.
What is the requirement for controlling fire?
Answer:
To control fire, the requirement is to cool the materials on fire to a temperature below their ignition temperature and to disrupt their contact with air and thereby cut off the supply of oxygen.
Question 6.
How does water control fire?
Answer:
Water cools the combustible material so that its temperature is brought below its ignition temperature. This prevents the fire from spreading. Water vapours also surround the combustible material, helping in cutting the supply of air. So, the fire is extinguished.
Question 7.
When is water used to extinguish the fire?
Answer:
Water is used to extinguish the fire in case the burning materials are solids or liquids heavier than water.
![]()
Question 8.
What are the types of combustion? Explain them.
Answer:
There are two types of combustion:
- Rapid combustion
- Spontaneous combustion
1. Rapid combustion. Combustion that takes place at a very fast rate is called rapid combustion. In this type of combustion, both heat and light are released. Examples are burning of LPG, petrol, dry grass, matchstick, magnesium ribbon, etc.
2. Spontaneous combustion. Combustion that occurs without the aid of any external heat is known as spontaneous combustion. For Example, sodium and white phosphorus catch fire without any external heat.
Question 9.
What is an explosion?
Answer:
When a sudden reaction takes place with the evolution of heat, light and a large amount of gas then the reaction is called an explosion. Examples of explosions are the bursting of crackers and the shot of a gun.
Question 10.
What is a flame?
Answer:
A flame is a zone where the burning of gases or vapour takes place with the production of light and heat. A flame results due to rapid combustions of fuel.
![]()
Question 11.
What is the colour of a candle flame?
Answer:
The candle flame is of blue colour.
Question 12.
Draw and explain the different zones of the candle flame.
Answer:
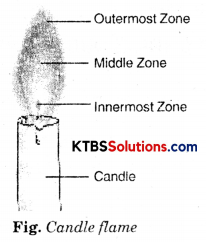
A candle flame has mainly three zones:
(i) The innermost zone: The innermost zone of the flame is cooler than the other zones and it is dark. It contains the fuel (wax) in the gaseous state.
(ii) The middle zone: This is the largest zone of the flame. It is also the bright zone. In this zone, the fuel burns partially and forms carbon particles. The hot carbon particles emit light. This zone gives soot and smoke.
(iii) The outermost zone: This zone of the flame is thin and blue in colour. As the oxygen from the air is readily available in this zone, there is complete combustion of the fuel. This is the hottest zone of the flame. The temperature of this zone is maximum around 1800°C.
Question 13.
What is observed when a clean glass plate is introduced into the luminous part of the flame? What does it indicate?
Answer:
A circular blackish ring is formed on the glass plate. It indicates the deposition of unburnt carbon particles present in the luminous zone of the flame.
![]()
Question 14.
Which part of the candle flame is the hottest?
Answer:
Non-luminous zone.
Question 15.
Name few fuels.
Answer:
Wood, charcoal, petrol, kerosene.
Question 16.
Write few qualities of fuels.
Answer:
Fuel is a substance which is readily available. It is cheap. It burns easily in the air at a moderate rate. It produces a large amount of heat. It does not leave behind any undesirable substance.
Question 17.
What is the calorific value of fuel? What is its unit?
Answer:
The amount of heat energy produced on complete combustion of 1kg of a fuel is called its calorific value. The calorific value of a fuel is expressed in a unit called kilojoules per kg (kJ/kg).
![]()
Question 18.
What is global warming?
Answer:
Global warming is the rise in the temperature of the environment of the earth. This results in the melting of polar glaciers. This leads to rising in sea level, causing floods on the sea coast.
Question 19.
How is acid rain harmful?
Answer:
Acid rain is harmful to crops, buildings, and soil.
Question 20.
Which fuel replaces the use of diesel and petrol as fuels in automobiles? Why?
Answer:
CNG. CNG produces pollutants in very small amounts.
Activities
Activity 1.
Collect some materials like straw, matchsticks, kerosene oil, paper, iron nails, stone pieces, glass, etc.
Under the supervision of your teacher try to burn each of these materials one by one. If combustion takes place mark the material combustible, otherwise mark it non-combustible (Table)
Table: Combustible and Non-combustible Substances
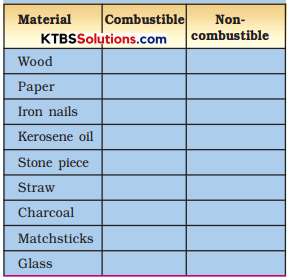
Can you name some more substances which are combustible?
Answer:
Coal, bamboo, clothes, rubber, plastic, etc. are combustible substances.
![]()
Activity 2.
Fix a lighted candle on a table. Put a glass chimney over the candle and rest it on a few wooden blocks in such a way that air can enter the chimney [Fig. (a)]. Observe what happens to the flame. Now remove the blocks and let the chimney rest on the table [Fig. (b)]. Again observe the flame. Finally, put a glass plate over the chimney [Fig. (c)]. Watch the flame again. What happens in the three cases? Does the flame flicker off? Does it flicker and give smoke? Does it burn unaffected? Can you infer anything at all about the role played by air in the process of burning?
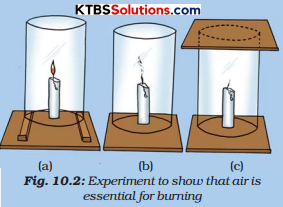
Answer:
We find that for combustion, the air is necessary. The candle burns freely in case (a) when air can enter the chimney from below. In case (b), when air does not enter the chimney from below, the flame flickers and produces smoke. In case (c), the flame finally goes off because the air is not available. Hence air is necessary for the process of burning.
Activity 3.
Place a piece of burning wood or charcoal on an iron plate or Tawa. Cover it with a glass jar or a tumbler, or a transparent plastic jar. Observe what happens. Does charcoal stop burning after some time? Can you think of the reason why it stops burning?
Answer:
First, the coal goes on burning, but it stops burning as soon as the glass jar, tumbler, or transparent plastic jar is placed on it. It cuts off the supply of air; which is necessary for burning anything.
![]()
Activity 4.
Make two paper cups by folding a sheet of paper. Pour about 50 ml of water into one of the cups. Heat both the cups separately with a candle (Fig.).
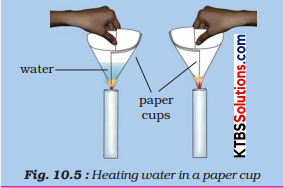
Now answer the following questions:
Question (i).
What happens to the empty paper cup?
Answer:
Empty paper cup starts burning.
Question (ii).
What happens to the paper cup with water?
Answer:
Paper cup with water does not burn.
Question (iii).
Does water in this cup become hot?
Answer:
Yes.
![]()
Question (iv).
Why does a paper cup with water does not burn?
Answer:
The heat supplied to the paper cup is transferred to the water lowers the temperature of the paper. In the presence of water, the ignition temperature of paper is not reached. Hence, it does not burn.
Activity 5.
Hold a glass tube with a pair of tongs and introduce its one end in the dark zone of a non-flickering candle flame (Fig.) Bring a lighted matchstick near the other end of the glass tube.

Question (i).
Do you see a flame? If so, what is it that produces a flame?
Answer:
Yes, the molten wax (of the candle) rises through the wick and is vaporized during burning forming a flame.
Question (ii).
In activity 5, could the vapours of wax coming out of the gas tube be the cause of the flame produced?
Answer:
Yes, it is so.
![]()
Question (iii).
Does it indicate that the blue non-luminous zone of the flame has a high temperature?
Answer:
Yes, it is.