You can Download Chapter 9 Biomolecules Questions and Answers, 1st PUC Biology Question Bank with Answers, Karnataka State Board Solutions help you to revise complete Syllabus and score more marks in your examinations.
Karnataka 1st PUC Biology Question Bank Chapter 9 Biomolecules
1st PUC Biology Biomolecules NCERT Text Book Questions and Answers
Question 1.
What are macromolecules? Give examples.
Answer:
Chemical compounds with molecular weights more than one thousand dalton and found in the acid insoluble fraction are called macromolecules. Eg: Polysaccharides, nucleic acids.
Question 2.
Illustrate a glycosidic, peptide and a phosphodiester bond.
Answer:
(a) Glycosidic bond: It is a bond formed between two monosaccharide molecules in a polysaccharide. This bond is formed between two carbon atoms of two adjacent monosaccharides.
(b) Peptide bond: Amino acids are linked by a peptide bond which is between the carbonyl (- COOH) group of one amino acid and amino (- NH2) group of the next amino acid which is formed by dehydration process.
(c) Phosphodiester bond: This is the bond present between the phosphate and hydroxyl group of sugar which is called an ester bond. As this ester bond is present on either side, it is called phosphodiester bond.
![]()
Question 3.
What is meant by tertiary structure of proteins”
Answer:
The long protein chain which is folded upon itself like a hollow woolen ball, is called tertiary structure of protein. It is necessary for many biological activities of proteins.
Question 4.
Find and write down structures of 10 interesting small molecular weight biomolecules. Find if there is any industry which manufactures the compounds by Isolation. Find out who are the buyers.
Answer:
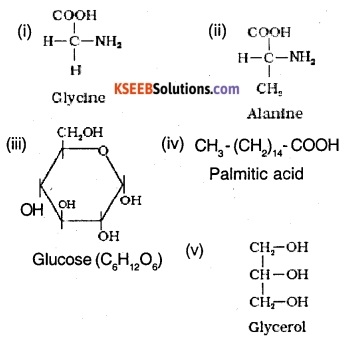
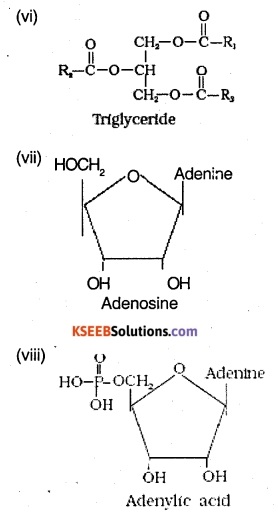
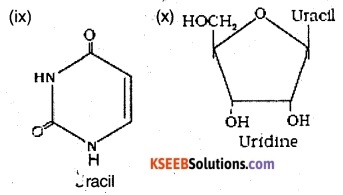
Ranboxy, Merck etc. manufacture these micromolecules. Research institutes by these micromolecules
Question 5.
Proteins have primary structure, if you are given a method to know which amino acid is at either of the two termini (ends) of a protein, can you connect this information to purity or homogeneity of a protein?
Answer:
Yes, if we are given a method to know the sequence of proteins, we can connect this information to the purity of a protein. Accurate sequence1 of a certain amino acid is very important for the functioning of protein. Any change in the sequence can be linked to the purity or homogeneity of a protein.
![]()
Question 6.
Find out and make a list of proteins used as therapeutic agents. Find other applications of proteins (e.g., Cosmetics etc.)
Answer:
Haemoglobin, Insulin, thyroxine, growth hormone, other hormones of adeno hypophysis, serum albumen, serum globulin, fibrinogen etc. are used as the therapeutic agents. Proteins are also used for synthesis of food supplements, film, paint, plastic etc.
Question 7.
Explain the composition of triglyceride.
Answer:
Glycerol combines with three similar fatty acids to form triglyceride. Structure/Composition of triglyceride

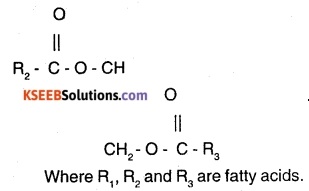
Question 8.
Can you describe what happens when milk is converted into curd or yoghurt, from your understanding of proteins?
Answer:
Conversion of milk into curd is the digestion of milk protein casein. Semi digested milk is the curd. In the stomach, renin converts milk protein into paracasein which then reacts with Ca++ ion to form calcium paracaseinate which is called the curd or yoghurt.
Question 9.
Draw the structure of the amino acid, alanine.
Answer:
Structure of alanine

Question 10.
What are gums made of? Is Fevicol different?
Answer:
Gums are polymeric substances which come under secondary metabolites of plants. Fevicol is a synthetic gum.
Question 11.
Find out a qualitative test for proteins, fats and oils, amino acids and test any fruit juice, saliva, sweat and urine for them.
Answer:
(a) Test for protein
- Xanthoproteic test
- Biuret’s test
(b) Test for Amino acid
- Ninhydrin test
(c) Test for fats and oils
- Sudan test
- Spot test
Question 12.
Describe the important properties of enzymes.
Answer:
Properties of enzymes
- An enzyme like any protein has primary structure (amino acid sequence). Secondary structure and tertiary structure
- Every enzyme has an ’active site’ which is a crevice into which the substrate fits.
- Enzymes are specific in their action. Particular enzyme acts on a particular substrate. But different enzymes catalyse a unique chemical or metabolic reaction.
- Enzymes generally function in a narrow range of temperature and pH. Activity declines both below and above optimum temperature and pH.
- The higher the affinity of the enzyme for its substrate the greater is its catalytic activity.
- The activity of an enzyme is also sensitive to the presence of specific chemicals that bind to the enzyme. For eg: Inhibitors that shuts off enzyme activity and
Co-factors that facilitates catalytic activity. - Enzymes retain their identity at the end of the reaction.
1st PUC Biology Biomolecules Additional Questions and Answers
1st PUC Biology Biomolecules One Mark Questions
Question 1
What are biomolecules?
Answer:
Biomolecules or biological,molecules are the elements occurring in the molecular form, in the living systems.
Question2.
Name the biomolecules of life.
Answer:
Carbohydrates, Lipids, Proteins, Enzymes and nucleic acids.
Question 3.
What are 3 groups of Carbohydrates?
Answer:
- Monosaccharides
- Oligosaccharides
- Polysaccharides.
Question 4.
What is a Glycosldlc bond?
Answer:
The bond formed between two monosaccharides is called glycosidic bond.
Question 5.
Name the disaccharide present in milk.
Answer:
Lactose.
Question 6.
Name the bond formed between sugar molecules.
Answer:
Glycosidic bond.
Question 7.
What are Polysaccharides?
Answer:
Polysaccharides are the complex carbohydrates and are the polymers of simple sugar molecules.
![]()
Question 8.
Name the Polysaccharide present in the plant cell wall. (U. Kannada 2008)
Answer:
Cellulose.
Question 9.
Name the Polysaccharide present in human liver.
Answer:
Glycogen.
Question 10.
Define Protein.
Answer:
Proteins are the microbiological molecules composed of monoacids.
Question 11.
What are the monomeric units of proteins?
Answer:
Aminoacids.
Question 12.
Where does histone occur?
Answer:
Chromosomes.
Question 13.
Name the proteins of hairs and nails.
Answer:
Keratin.
Question 14.
Give an example for simple protein.
Answer:
Albumin.
Question 15.
What is a peptide bond? (Gulbarga 2004)
Answer:
It is a bond formed between two amino acids in a protein molecule. (CO-NH)
Question 16.
Give an example for Conjugated proteins.
Answer:
Glycoprotein (Bijapur. 2004, B.North. 04)
Question 17.
Give an example for derived proteins.
Answer:
Peptone.
Question 18.
Give an example for nonessential amino acids?
Answer:
Glutamic acid.
Question 19.
Define lipid.
Answer:
Lipids are the organic compounds composed of carbon, hydrogen and oxygen as well. as nitrogen and phosphorus. They are the esters of fatty acids and alcohol.
Question 20.
Give an example of simple lipid.
Answer:
Waxes.
Question 21.
Name two unsaturated fatty acids.
Answer:
Ole’s acid and Linoleic acid.
Question 22.
Name two saturated fatty acids.
Answer:
Butyric acid, Stearic acid.
Question 23.
Give an example for phospholipids.
Answer:
Lecithin.
Question 24.
What is an enzyme? (B’lore North 2004)
Answer:
Enzymes are biological catalysts produced by living cells.
Question 25.
What you mean by the activation of enzymes?
Answer:
The conversion of inactive form of an enzyme into active form is known as activation of enzymes.
Question 26.
What are Lyases?
Answer:
These are the enzymes that catalyse reactions involving breaking of large molecules without the addition of water.
Question 27.
What are Hydrolases ?
Answer:
These are enzymes capable of accelerating hydrolytic reactions.
Question 28.
What is an apoenzyme ?
Answer:
The protein part of an enzyme is called ‘apoenzyme’.
Question 29.
What is a Coenzyme?
Answer:
The nonprotein part of an enzyme is called ‘coenzyme’.
![]()
Question 30.
What Purines and Pyrimidines?
Answer:
These are nitrogenous bases.
Question 31.
What is a nucleoside?
Answer:
It is a combination of nitrogenous base and pentose sugar.
Question 32.
What Is a nucleotide? (Chikmagalur 2004)
Answer:
A nucleotide is a combination of nucleoside and phosphoric acid.
Question 33.
What is the bond present between the successive nucleotides of a single Poly-nucleotide strand?
Answer:
Phosphodiester.
Question 34.
What is the type of sugar present in RNA?
Answer:
Ribose sugar.
Question 35.
Give the name for animal starch.
Answer:
Glycogen. (Bijapur 2004)
Question 36.
Expand DNA. (Dharwar 2004)
Answer:
Deoxyribose Nucleic Acid.
Question 37.
Give an example for chromoproteins.
Answer:
Hemoglobin. (Gulbarga 2004)
Question 38.
What type of sugar is found in DNA?
Answer:
Deoxyribose (Belgaum 2004)
Question 39.
Give an example for asteroid. (B. North 2005)
Answer:
Testosterone, Progesterone
Question 40.
Define enzyme. (Tumkur 2005)
Answer:
Enzymes are biological catalysts produced by living cells.
Question 41.
What are triglycerides? (Udupi 2005)
Answer:
These are the esters of three molecules of fatty acids and one molecule of glycerol.
Question 42.
Mention one use of gelatin. (B1 North 2005)
Answer:
Gelatin is used in preparation of ice cream, medicinal capsules, films and photographic papers, (anyone)
Question 43.
Find the odd member in the given group giving one reason. Sucrose, glucose, lactose, maltose. (Kolar 2005)
Answer:
Glucose because it is a monosaccharide, while all the others are disaccharides.
Question 44.
Give one example for Disaccharide.
Answer:
Sucrose, Lactose (D.Kannada 2007)
Question 45.
Name the catalytic proteins. (D.K. 2008)
Answer:
Pepsin, amylase esterases, polymerases.
Question 46.
Name the pyrimidine present only in DNA.
Answer:
Jhymne. (D.Kannada 2010)
Question 47.
What is meant by ash?
Answer:
Ash is the remnant of a living tissue that is denied and fully burnt till all the carbon compounds in it is oxidized to gaseous form and removed.
Question 48.
What are amino acids?
Answer:
Amino acids are organic compounds containing an amino group and an acidic group as substituents on the same carbon (a – carbon)
Question 49.
How many types of amino acids are found in proteins?
Answer:
Twenty types.
Question 50.
Name any two aromatic amino acids?
Answer:
Tyrosine, tryptophan.
![]()
Question 51.
Why do amino acids change their structure in varying pHs?
Answer:
Because of the ionizable nature of – NH2 and – COOH groups present in amino acid, structure of amino acids changes in solutions of different pHs.
Question 52.
Give the zwitterionic form of amino acids.
Answer:

Question 53.
How many carbon atoms are present in each of the following:
(a) Palmitic acid
(b) Arachidonic acid?
Answer:
(a) 16 carbon atoms
(b) 20 carbon atoms.
Question 54.
Name the lipid that is tri-hydroxy propane.
Answer:
Glycerol.
Question 55.
Give examples of carbon compounds with heterocyclic rings?
Answer:
Adenine, guanine, cytosine, uracil, and thymine.
Question 56.
Name the nitrogen base present only in RNA?
Answer:
lilac
Question 57.
What is polymer of amino acids called?
Answer:
Proteins.
Question 58.
Mention the function of GLUT – 4?
Answer:
It enables glucose transport into cells.
Question 59.
Give few examples of alkaloids.
Answer:
Morphine and Codeine.
Question 60.
Give examples of toxins.
Answer:
Ricin and Abrin.
Question 61.
What is the function of collagen?
Answer:
It forms the intercellular ground substance.
Question 62.
Give the name of proteins that fight infectious agents.
Answer:
Antibodies.
Question 63.
Name the protein that is most abundant in animal world.
Answer:
Collagen.
Question 64.
Name the protein that is most abundant in whole of biosphere.
Answer:
Ribulose bisphosphate Carboxylase – Oxygenase (Ru Bis Co).
Question 65.
Mention the polymer of fructose.
Answer:
Inulin.
Question 66.
Why cannot cellulose hold l2 molecules?
Answer:
Cellulose does no contain complex helices and hence cannot hold l2 molecules.
Question 67.
Name the chemical present in the exoskeleton of arthropods.
Answer:
Chitin.
![]()
Question 68.
Give examples for heteropolysaccharide.
Answer:
Glucosamine, Chitin.
Question 69.
Name a few substituted purines.
Answer:
Adenine and guanine.
Question 70.
Name few substituted pyrimidines.
Answer:
Cytosine, Thymine and Uraciltion of carbonic acid from carbon dioxide and water.
Question 71.
How are the two strands of DNA held together?
Answer:
They are held by hydrogen fonds between the nitrogen bases.
Question 72.
How many hydrogen bonds exist between Guanine and Cytosine.
Answer:
Three.
Question 73.
How many hydrogen bonds exists between Adenine and Thymine?
Answer:
Two.
Question 74.
Define metabolism.
Answer:
Metabolism is all the chemical reactions that constantly occur in living organisms.
Question 75.
What is dynamic state of the body constituents?
Answer:
The flow of metabolites at a definite rate; and direction in the living body is called dynamic state of the body constituents.
Question 76.
What are enzymes chemically?
Answer:
Enzymes are chemically proteins.
Question 77.
Define the rate of chemical process and how is it expressed?
Answer:
Rate of a physical or chemical process refers to the amount of product formed per unit time. It is expressed as
\(rate=\cfrac { \delta P }{ \delta T } \)
Question 78.
What is the rule of thumb in the variation of rate?
Answer:
Rate doubles or decreases by half in either direction for every 10°C change in temperature.
Question 79.
What is active site’ of an enzyme?
Answer:
Active site of an enzyme is a service or pocket into which the substrate fits.
Question 80.
What is the function of carbonic anhydrase?
Answer:
Carhonic anhydrase catalyses the formation of carbonic acid from carbon dioxide and water
Question 81.
What is a substrate In an enzyme action?
Answer:
The chemical substance on which the enzyme acts is called the substrate.
Question 82.
Define activation energy of reaction.
Answer:
The difference in the average energy content of the substrate form, from that of the transition state is called activation energy.
Question 83.
What is an inhibitor of an enzyme action?
Answer:
The chemical substance which decreases or shuts off enzyme activity is called an inhibitor.
Question 84.
What is the essential components of many coenzymes? Give examples.
Answer:
Essential components of many coenzymes are vitamins, e.g.: coenzyme nicotinamide adenine dinucleotide (NAD) and NADP contain the vitamin niacin.
1st PUC Biology Biomolecules Two Marks Questions
Question 1.
Give a list of important monosaccharides.
Answer:
Glycerol, Ribose, Deoxyribose, Glucose, Fructose and Galactose.
Question 2.
What are the monosaccharides present in DNA and RNA? (Chikmagalur 2004)
Answer:
Deoxyribose in DNA and Ribose in RNA.
Question 3.
What are Polysaccharides ?Give examples. (Belgaum 2004)
Answer:
Polysaccharides are the polymers of simple sugar molecules. They contain more than 10 units of monosaccharides. Examples: Starch, Cellulose.
Question 4.
Write two functions of carbohydrates.
Answer:
- They are the principal source of energy.
- They form the structural components in living organisms.
Question 5.
In which form carbohydrate Is stored in plants and animals?
Answer:
- plants – starch.
- In animals – Glycogen.
Question 6.
What are homopolysaccharides ? Give two examples.
Answer:
These are polysaccharides composed of only one type of monosaccharides.
Examples: Starch, Cellulose.
Question 7.
What la a prosthetic group? Give an example.
Answer:
The non protein part of a conjugated protein is called a prosthetic group. For example in a nucleoprotein (nucleic acid is the prosthetic group).
Question 8
List any two functions of proteins.
Answer:
Proteins act as biocatalyst as they form enzymes. Proteins like fibrinogens help in the clotting of blood.
Question 9.
What la an aminoacid sequence?
Answer:
It is a particular order in which amino acids are arranged to form a specific protein. The aminoacids sequence in a protein molecule is of paramount importance.
Question 10.
What are saturated and unsaturated fatty acids? Give examples. .
Answer:
- Saturated fatty acids do not possess double bonds in their hydrocarbon chain.
Examples: butyric acid. - Unsaturated fatty acids have one or more double bonds in their hydrocarbon chain. Example: Oleic acid.
Question 11.
Write two functions of Lipids. (B. North 04)
Answer:
’They function as reserve food material in living organisms. They provide a rich source of energy.
Question 12.
What are enzymes? Mention any 2 groups of enzymes.
Answer:
Enzymes are the stable, specific protein molecules which act as biocatalysts.
Following are the 2 groups of enzymes:
- Oxide – reductases
- Isomerases.
Question 13.
What are the components of enzymes?
Answer:
Enzymes are made up of protein as well as non – protein part. The protein part is called apoenzyme and the non-protein part is coenzyme. These two together are called holoenzyme.
Question 14.
State the components of DNA.
Answer:
DNA is composed of nitrogenous bases like adenine, guanine, cytosine and Thymine, pentose sugar deoxyribose and phosphate group.
Question 15.
Name the nitrogenous bases of RNA. (Shimoga 2006)
Answer:
- Adenine
- Guanine
- Cytosine
- Uracil
Question 16.
Write any two functions of DNA.
Answer:
- It controls protein biosynthesis.
- It is the genetic material in most of the organisms.
Question 17.
What is a nucleotide? List the nucleotides of RNA.
Answer:
A nucleotide is the combination of nitrogenous base, sugar and phosphoric acid. The following are the nucleotides of RNA.
- adenosine monophosphate (AMP)
- guanosine monophosphate (GMP)
- cytidine monophosphate (CMP)
- Uridine monophosphate (UMP)
Question 18.
What are the functions of RNA.
Answer:
- It acts as genetic material in many viruses.
- It helps in the process of protein synthesis.
![]()
Question 19.
List any two differences between DNA and RNA. (B. North., Gulbarga. 2004, D.K. 2006,2008)
Answer:
- DNA is a double-stranded molecule. RNA is a single-stranded molecule.
- DNA has deoxyribose sugar.
- RNA has Ribose sugar.
- DNA is genetic RNA is non-genetic
- DNA has Adenine, cytosine, Gnanine & Thymine as Nitrogenous bases RNA has Adenine, cytosine, Guanine & uracil as nitrogenous bases
Question 20.
Give an example for the following
(a) Respiratory pigment
(b) Protein present In hair
(c) Protein present in muscle fiber
(d) Sugar in milk.
Answer:
(a) Respiratory pigment: Haemoglobin.
(b) A protein present in hair: Keratin
(c) Protein present in muscle fibre: Myosin
(d) Sugar in milk: Lactose.
Question 21.
With reference to DNA, mention the types of
(a) Pyrimidine bases
(b) Purine bases (Udupi 2005)
Answer:
(a) Pyrimidine bases – Cytosine & Thymine
(b) Purine bases – Adenine & Guanine
Question 22.
Write four properties of enzymes (D.Kannada 2005,2011)
Answer:
- Enzymes are needed in a minute quantity for the acceleration of biochemical reactions.
- Enzymes exhibit specificity in their reactions. Each enzyme can act on a particular substance/substrate only.
- Enzymes act within narrow range of pH and temperature. So they are referred to as thermolabile compounds.
- Enzymes can act either way in a biochemical reaction, because biochemical reactions are reversible.
Question 23.
Differentiate between a nucleoside and a nucleotide. (Bangalore North 2005)
Answer:
Nucleoside
- Consists of sugar and nitrogenous base.
- The linkage present is called glyosidic.
- Eg. is Adenosine, Deoxyadenosine.
Nucleotide
- Consists of sugar, nitrogenous base & phosphoric acid.
- The linkage present is termed phosphorites linkage.
- Eg. is Adenylic acid, deoxy adenylic acid.
Question 24.
Give any two biological significances of amino acids. (Udupi 2005)
Answer:
- Some proteins act as chemical messengers (hormones).
- Muscle protein like actin and myosin help in muscle contraction.
Question 25.
Name the monomer of nucleic acids and mention Its components. (M.Q.P, Kolar, Hassan, Shimoga, Belgaum, Bangalore Rural,Chikmagalur 2005)
Answer:
Nucleotide comprising sugar, nitrogenous base and phosphoric acid.
Question 26.
Give the biological significance of carbohydrates. (Bangalore Rural 2005)
Answer:
- Carbohydrates are the major source of energy for the living world.
- They form the reserve food material in both plants and animals.
- They form the structural components in living organisms.
- They are essential in the regulation of fat metabolism.
Question 27.
Write any five biological significance of lipids. (Bangalore South 2005)
Answer:
- lipids form the main constituents of biological membranes.
- Lipids provide protective coverings like cuticle on leaves, hairs & feathers in animals.
- Lipids function as reserve food material in living organisms.
- Lipids provide a rich source of energy.
- Steroids regulate physiological activities.
Question 28.
Write the structural formula of:
(I) Galactose
(II) glucose
Answer:
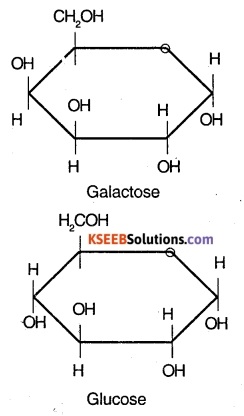
Question 29.
What are fatty acids? Give two examples.
Answer:
Fatty acids are compounds which has a carboxyl group attached to an R group. The R group could be a methyl (- CH3), or ethyl (- C2Hs) or higher number of – CH2 groups (1 carbon to 19 carbons). Eg; Palmitic acid, Arachidonic acid.
Question 30.
State the differences between oils and fats
Answer:
| Oils | Fats |
| (a) Oils are liquids at temperature. (b) Melting point is low (c) They are unsaturated compounds (d) They react easily |
(a) Fats are solids at room temperature. (b) Melting point is high (c) They are saturated (d) They do not react easily. |
Question 31.
Differentiate between essential and non essential amino acids.
Answer:
| Essential amino acids | Non-essential amino acids |
| (a) These are the amino acids that are not synthesized in the body. | (a) These are the amino acids that are synthesized in the body. |
| (b) Dietary proteins form the source of essential amino acids. | (b) They need not be taken in the diet. |
Question 32.
Differentiate between starch and cellulose.
Answer:
| Starch | Cellulose |
| (a) It forms helical secondary structures (b) Starch can hold l2 molecules (c) Starch is stained blue with iodine (d) It is a storehouse of energy in plant tissues |
(a) It does not contain helical structures. (b) It cannot hold l2 molecules (c) It cannot be stained blue with iodine (d) It is present in the cell wall |
Question 33.
Write the structural formula of
(i) Adenylic acid
(ii) Uracil
Answer:
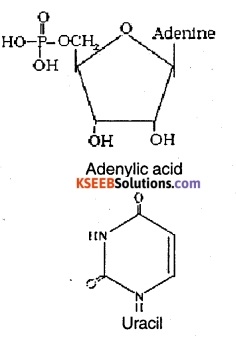
Question 34.
Describes the primary structure of proteins.
Answer:
The sequence of amino acids, i.e. the positional information of the amino acids in a protein is called the primary structure of a protein. A Protein is imagined as a line, the left end represented by the first amino acid and the right end represented by the last amino acid. The first amino acid is also called as N-terminal amino acid and the last amino acid is called the C-terminal amino acid.
Question 35.
Describe the quaternary structure of proteins and give example.
Answer:
Some proteins are an assembly of more than one polypeptide or subunits. The manner in which these individual folded polypeptides or subunits are arranged with respect to each other (e.g. linear string of spheres, spheres arranged one upon the other in the form of a cube or plate) is the architecture of a protein which is referred as the quaternary structure of a protein. Eg: Adult human haemoglobin consists of 4 subunits. Two of these are identical to each other. Hence, two subunits of a -type and two subunits of p -type together constitute the human haemoglobin (Hb).
![]()
Question 36.
What are cofactors for enzymes? Name the types of cofactors.
Answer:
The non-protein constituents that are found to the enzyme to make it catalytically active are called as co-factors. Types of co-factors are
- prosthetic group
- Co-enzymes
- Metal ions.
1st PUC Biology Biomolecules Three Marks Questions
Question 1.
Explain the terms monoglyceride, diglyceride and triglyceride.
Answer:
- Monoglyceride: It is a lipid with one molecule of glycerol and one molecule
of fatty acid. - Diglyceride: It is a lipid with one molecule of glycerol and two molecules
of fatty acids. - Triglyceride: It is a lipid that has one molecule of glycerol and three molecules
of fatty acids.
Question 2.
What are nucleosides? Name the four of them which occur in RNA.
Answer:
Nucleosides are compounds having nitrogen bases attached to a sugar.
Nucleosides occurring in RNA are:
- Adenosine
- guanosine
- cytidine
- and uridine.
Question 3.
Mention the differences between primary and secondary metabolites. Give examples of each.
Answer:
| Primary Metabolite | Secondary Metabolites |
| (a) These are the metabolites which have identifiable functions and play a specific role in metabolism. (b) These are important in human metabolic processes Eg: amino acids, sugars. |
(a) These are the metablites formed during metabolism whose exact function is not known. (b) These are useful for human welfare Eg: Alkaloids, flavonoids antibiotics. |
Question 4.
Name the two types of biomolecules present in the cells of living organisms. Mention the differences.
Answer:
Two types of biomolecules are
| Biomacromolecules | Biomacromolecules |
| (a) These molecules have their molecular weight less than one thousand daltons. (b) These are found in the acid-soluble fraction. Eg: amino acids, sugars, alkaloids, etc |
(a) These biomolecules have molecular weight in the range of ten thousand daltons and above. (b) These are found in the acid-insoluble fraction. Eg: proteins, nucleic acids polysaccharides, etc. |
Question 5.
(a) What is meant by complementary base pairing?
(b)What is the distance between two successive bases in a strand of DNA?
(c)How many base pairs are present in one turn of the helix of a DNA strand?
Answer:
(a) In a DNA, a purine always pairs with a pyrimidine i.e. adenine pairs with thymine
(A = T) and guanine with cytosine (G = C). This is called a complementary pairing.
(b) 3.4 A0
(c) 10 base pairs.
Question 6.
Describe the influence of temperature and pH on enzyme action with graphs.
Answer:
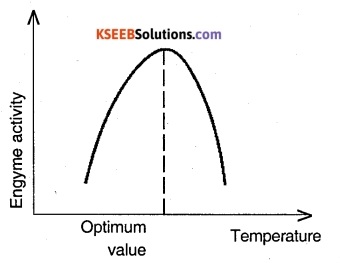
Enzymes generally function in a narrow range of temperature and pH. Each enzyme shows its highest activity at a particular temperature and pH called the optimum temperature and optimum pH. Activity declines both below and above the optimum value. Low temperature preserves the enzyme in a temporarily inactive state whereas high temperature destroys enzymatic activity because proteins are denatured by heat. Any change in pH alters the tertiary structure of the enzyme protein and thereby reduces the enzyme activity.
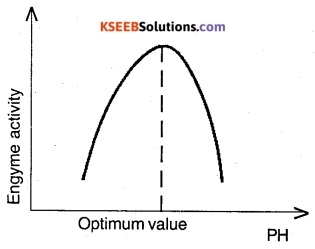
Question 7.
Describe competitive inhibition with an example.
Answer:
When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as a competitive inhibitor and the process is called competitive inhibition. Due to its close structural similarity with the substrate, the inhibitor competes with the substrate for the substrate-binding site of the enzyme. Consequently, the substrate cannot bind and as a result, the enzyme action declines.
Eg:- Inhibition of succinic dehydrogenase by malonate which closely resembles the substrate succinate in structure.
Question 8.
How many classes are enzymes divided into? Name all the classes.
Answer:
Enzymes are divided into 6 classes. Namely
(a)Oxidoreductases/dehydrogenases:
Enzymes which catalyse oxidoreduction between two substrates
(b)Transferases:
Enzymes catalysing a transfer of group between a pair of substrate.
(c) Hydrolases:
Enzymes catalysing hydrolysis of ester, ether, peptide, glycosidic, C-C-C-halide or P.N bonds.
(d) Lyases:
Enzymes that catalyse removal of groups from – substrates by mechanisms other than hydrolysis leaving double bonds.
(e) Lyases:
Enzymes catalysing inter conversion of optical geometric or positional isomers.
(f) Ligases:
Enzymes catalysing the linking together of 2 compounds.
1st PUC Biology Biomolecules Five Marks Questions
Question 1.
Enumerate the properties of enzymes and ” its classification. (D. Kannada 2007)
Answer:
Properties of Enzymes:
- Enzymes are needed in a minute quantity for the acceleration of biochemical reactions.
- Enzymes exhibit specificity in their reactions. Each enzyme can act on a particular substance only.
- Enzymes act within a narrow range of pH and temperature. So they are referred to as thermolabile compounds.
- Enzymes can act either way in a biochemical reaction because biochemical reactions are reversible.
- Enzymes can be destroyed or inactivated by a number of substances like cyanide, iodo acetic acid, malonic acid, etc. The substances capable of hampering enzymatic reactions are called enzyme inhibitors.
- Enzymes work in teams, making cell metabolism a continuous process.
Classification of enzymes.
‘Enzymes are classified into six major groups based on type of functions.
They are –
- Oxide-Reductases
- Transferases
- Hydrolases
- Lyases
- Isomerases
- Ligases
(1) Oxide-Reductases:
These are enzymes catalysing oxidation and reduction reactions. These oxidation and reduction reactions are generally referred to as redox reactions.
(2) Transferases:
These enzyme accelerate reactions involving transfer of functional groups from one compound to another compound.
(3) Hydrolases:
These are enzymes capable of accelerating hydrolytic reactions. Breaking down of large molecules with the addition of water is called hydrolysis. Enzymes involved in hydrolytic reaction are referred to as hydrolases.
(4) Lyases:
These enzymes catalyse reactions involving breaking of large molecules without addition of water.
(5) Isomerases:
These enzymes catalyse reactions involving rearrangement of molecule structure of substrate to form an isomer. Hence they catalyse isomerisation reactions they are termed as isomerases.
(6) Ligases: These enzymes accelerate synthetic reactions like polymerisation.
Question 2.
a. Expand DNA. (D.Kannada 2006)
Answer:
Deoxy ribo nucleic acid
b. List the nucleotides of DNA
Answer:
d AMP (deoxy adenylic acid)
d GMP (deoxy guanylic acid)
d CMP (deoxy cytidylic acid)
d TMP (deoxy thymidylic acid)
c. Write 4 functions of DNA.
Answer:
- Carries genetic information
- Auto catalytic and heterocatalytic function (replication and protein synthesis)
- Mutation
- Repair
Question 3.
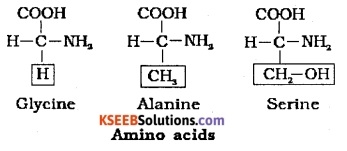
What will be the amino acid if the R-group is:
(i) hydrogen
(ii) methyl group
(iii) hydroxymethyl group write the respective structures
Answer:
(i) Glycine
(ii) Alanine
(iii) Serine
Question 4.
Mention any five secondary metabolites with two examples for each.
Answer:
- Pigments – Carotenoids, Anthocyanins
- Alkaloids – Morphine, Codeine etc.
- Essential oils – Lemon grass oil
- Toxins – Abnn, Ricin
- Drags – Vinblastin, curcumin
- Polymeric substances – Rubber, gems
- Terpenoids – Monoterpenes, Diterpenes.
Question 5
Mention any five proteins with their functions.
Answer:
- Collagen – Intercellular ground substance
- Trypsin – Enzyme
- Insulin – Hormone
- Antibody – Fights infectious agents
- Receptor – Sensory reception like smell, taste, hormone etc.
- GLUT- 4- Enables glucose transport into cells.
Question 6.
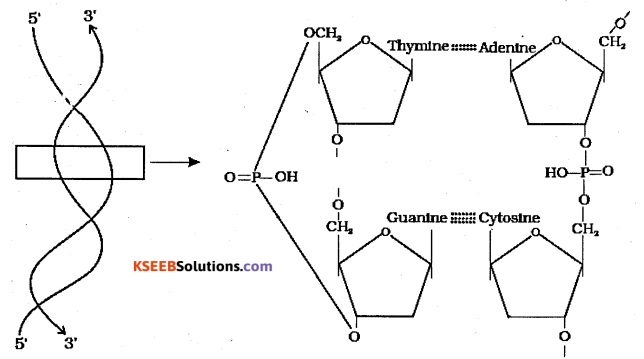
Describe the Watson and Crick model of DNA structure with a diagram.
Answer:
Watson and Crick model explains the secondary structure exhibited by DNA. This model says that the DA exists as a double helix. The two strands of polynucleotides are antiparallel Le. run in the opposite direction. The backbone is formed by the sugar-phosphate-sugar chain. The nitrogen bases are projected more or less perpendicular to this backbone but face inside.
‘A’ of one strand compulsorily base pairs with T on the other strand and ‘G’ of one strand pairs with ‘C’ on the other strand. Three are two hydrogen bonds between A and T and three hydrogen bonds between G and C. Each strand appears like a helical staircase. Each step of ascent is represented by a pair of bases. At each step
of ascent, the strand turns 360. One full turn of the helical strand would involve ten steps or ten base pairs. The pitch is 34A° and the rise per base pair is 3.4 A° The form of DNA with the above mentioned salient features is called B-DNA.
Question 7.
How do enzymes accelerate the biochemical reactions? Explain the nature of enzyme action, describing the steps In catalytic cycle of enzyme action.
Answer:
Enzymes accelerate the reaction by reducing the activation energy of the process/reaction. Each enzyme (E) has a substrate (S) binding site in its molecule so that a highly reactive enzyme-substrate complex (ES) is produced. This complex is short-lived and dissociates into its products (P) and the unchanged enzyme with an intermediate formation of the enzyme-product complex (EP). The formation of the ES complex is essential for catalysis.
E + S □ ES → EP → E + P
The catalytic cycle of enzyme action can be described in the following steps:
- The substrate binds to the active site of the enzyme, fitting into the active site.
- The binding of the substrate induces the ‘ enzyme to alter its shape, fitting more tightly around the substrate.
- The active site of the enzyme, now in close proximity of the substrate breaks the chemical bonds of the substrate and the new enzyme- product complex is formed.
- The enzyme releases the products of the reaction and the free enzyme is ready to bind to another molecule of the substrate and run through the catalytic cycle once again.
![]()
Question 8.
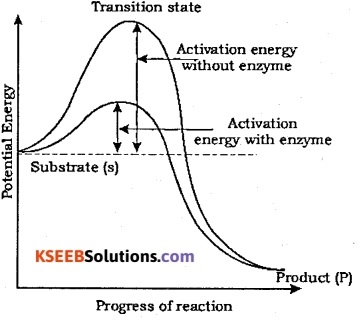
Explain how enzymes bring about high rates of chemical conversions using the concept of activation energy.
Answer:
Consider the following reaction in which ‘ the enzyme (E) that is a protein with an ‘active site’ converts a substrate (S) into a product (P)
![]()
Substrate ‘S’ binds the enzyme (E) at its active site within a given cleft or pocket to form an ‘ES’ complex. This complex formation is a transient phenomenon. During the state where substrate is found to the enzyme active site, a new structure of the substrate called transition state structure is formed. After the bond breaking/making is completed, the product ‘P’ is released from the active site.
Here the structure of substrate gets transformed into the structure of products (S). The pathway of this transformation goes through transition state structure and many more ‘altered structural states’ between the stable substrate and the product. Stability is related to the energy status of the molecule.
If product ‘P’ is at a lower level than ‘S’ (Refer the figure given below), the reaction is exothermic or spontaneous. However, whether it is an exothermic or endothermic (energy requiring) reaction, the ‘S’ has to go through a much higher energy state or transition state. The difference in average energy content of “S’ from that of this transition state is called activation energy. Enzymes bring down this energy barrier making the transition of ‘S’ to ‘P’ more easy and quicker.
Question 9.
Fill in the blanks.
(1) In a polysaccharide ……………is the reducing end and is the non-reducing end.
Answer:
Right end, left end
(2) Metabolic pathways that lead to complex from the simpler structure is called ………….. pathway.
Answer:
anabolic
(3) The most important form of energy in living systems is ……………….
Answer:
Adenosine triphosphate (ATP)
(4) In our skeletal muscle, under anaerobic conditions acid is formed and under aerobic conditions ………….. acid is formed.
Answer:
Lactic, Pyruvic